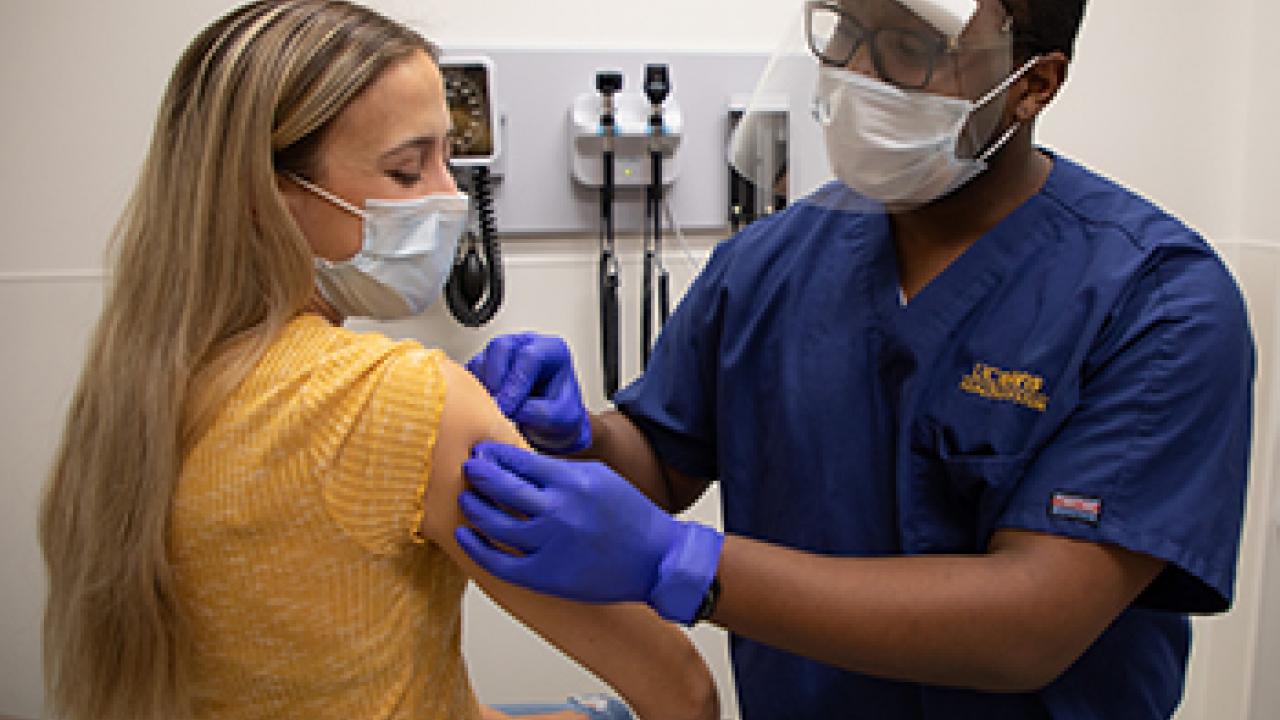
UC Davis Health launches new COVID-19 vaccine trial
Research into additional coronavirus vaccines continues across the country, including at UC Davis Health in Sacramento.
The health system launched a Phase 3 clinical trial with the National Institutes of Health (NIH) and Novavax this week to test another experimental vaccine to help address the global pandemic.
UC Davis Health plans on enrolling 200-300 participants at its testing clinic site near UC Davis Medical Center. The overall research effort seeks to enroll up to 30,000 people in the U.S. and Mexico. It will prioritize participants from groups who are most affected by COVID-19, including Latino, African American and Native American communities.
The testing will be done as a “randomized, double-blinded trial.” Among the participants, there will be two people getting vaccine for every person receiving placebo. Neither the participants nor the researchers will know who gets the vaccine or who receives the placebo. The vaccine, which is done as an injection in the upper arm, will be given in two doses, 21 days apart.
Participants will be required to make 8-10 visits to the clinic during the estimated 26-month study. Participation also includes modest compensation and, potentially, reimbursement for travel. As with previous U.S. clinical trials for a COVID-19 vaccine, patients who receive the placebo will likely be prioritized for vaccination if it is authorized for use. Those interested in participating or finding out if they qualify can visit the study's webpage.
How the vaccine works
The Novavax vaccine, called NVX-CoV2373, has a subunit from the spike protein in SARS-CoV-2, the virus that causes COVID-19. The spike protein is the main target for development of immunity. The subunit is combined with an adjuvant, a boosting agent to improve the body’s immune response to the vaccine. When this combination enters the body, it triggers an immune response to the spike protein and creates antibodies to fight it.
The trial is led by Stuart Cohen, chief of the Division of Infectious Diseases and director of Hospital Epidemiology and Infection Prevention at UC Davis Health. He noted that this latest clinical trial also takes a natural infection approach. It means that study participants are not intentionally exposed to the virus.
“The [Novavax] vaccine contains protein antigens that cannot replicate or cause COVID-19. The antibodies generated by the vaccine will help protect the body from the real, fully-potent virus,” Cohen said recently.
Cohen’s research team plans to monitor the number of participants who naturally contract COVID-19 among the vaccinated and placebo groups. One indicator that the vaccine is working will be if there are substantially fewer infected participants in the vaccinated group than those who received the placebo.
Novavax and other COVID-19 vaccines
Currently, there are more than 100 vaccines under development globally. Two vaccines, Moderna and Pfizer, received emergency use authorizations from the federal Food and Drug Administration and are now being used in the first U.S. COVID-19 vaccinations programs, including at UC Davis Health.
The Moderna and Pfizer vaccines use what is called a “messenger RNA” model. The Novavax vaccine follows a more traditional vaccine model. It also has a critical advantage: It can be stored at temperatures between 36°F and 46°F (2°C to 8°C). This allows for a standard distribution of supplies, unlike the Pfizer and Moderna vaccines, which must be stored at subzero temperatures.
The Novavax trial is funded by a $1.7 million grant from the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) and sponsored by Fred Hutchinson Cancer Center.